When a patient switches from a brand-name HIV drug to a generic version, they expect the same results. But what if the generic doesn’t behave the same way in their body? This isn’t theoretical-it happens. And for drugs with a narrow therapeutic index, even small differences in absorption or metabolism can mean the difference between viral suppression and treatment failure. That’s where therapeutic drug monitoring (TDM) steps in-not as a routine check, but as a lifeline for patients on critical medications.
What Are NTI Drugs and Why Do They Need Special Care?
NTI stands for Narrow Therapeutic Index. These are drugs where the gap between an effective dose and a toxic one is razor-thin. A little too little, and the virus rebounds. A little too much, and the patient suffers liver damage, nerve pain, or even life-threatening toxicity. In HIV care, this applies most to protease inhibitors (like lopinavir, darunavir) and non-nucleoside reverse transcriptase inhibitors (like efavirenz, rilpivirine). These are the drugs that require precise blood levels to work.
Generic versions of these drugs are chemically identical on paper. But bioequivalence studies don’t capture everything. Differences in fillers, coating, or manufacturing processes can alter how fast or how much of the drug gets into the bloodstream. For most drugs, that’s fine. For NTI drugs? It’s risky. A 2022 study in the South African Journal of HIV Medicine found that patients on generic antiretrovirals who received TDM had 22% fewer treatment failures than those who didn’t. That’s not a small number-it’s lives saved.
How Therapeutic Drug Monitoring Actually Works
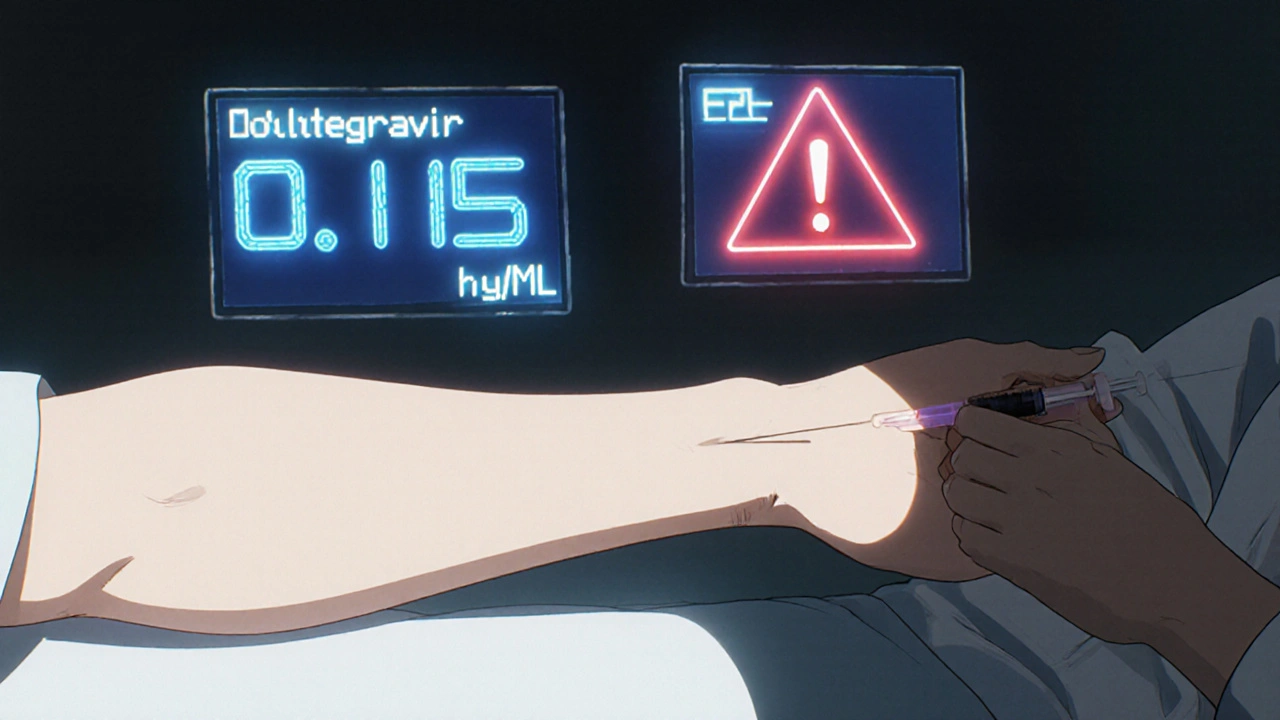
TDM isn’t just drawing blood and waiting. It’s a precise science. Clinicians measure the trough concentration-the lowest level of drug in the blood right before the next dose. This number must stay above the minimum inhibitory concentration (MIC) to suppress the virus, but below the threshold where side effects kick in.
For example, dolutegravir-a newer integrase inhibitor-has a target trough level of around 0.15 µg/mL. If a patient is taking rifapentine for tuberculosis (a common co-infection), that drug can drop dolutegravir levels by 26%. Without TDM, the clinician might assume the treatment is failing due to resistance. With TDM, they see the problem isn’t resistance-it’s a drug interaction. They adjust the dose, and within weeks, the viral load drops back to undetectable.
It’s not magic. It’s math. And it requires a lab with validated assays that can detect drug levels down to nanograms per milliliter. Not every hospital can do this. In the UK, only 3-5 specialized labs offer TDM for HIV drugs. In the U.S., private labs charge $450-$650 per test and deliver results in 2-3 days. Public health systems? Often 10-14 days. That delay can be deadly.
When TDM Makes Sense-and When It Doesn’t
TDM isn’t for every HIV patient. It doesn’t work for NRTIs (nucleoside reverse transcriptase inhibitors) like tenofovir or emtricitabine. Why? Because those drugs are prodrugs. They need to be activated inside cells, not measured in the blood. Testing plasma levels of tenofovir tells you nothing about what’s happening in the immune cells where the drug actually works.
So when should TDM be used? Only in specific, high-risk situations:
- Switching to a generic version of a protease inhibitor or NNRTI
- Patient has kidney or liver disease affecting drug clearance
- Drug interactions-like HIV meds with TB treatment, antifungals, or seizure drugs
- Poor absorption due to chronic diarrhea, gastric bypass, or other GI issues
- Pediatric patients or elderly patients with changing metabolism
- Unexplained treatment failure despite good adherence
The UK’s HIV guidelines explicitly list these as indications. The U.S. Department of Health and Human Services doesn’t recommend TDM routinely-but they don’t forbid it either. It’s a tool for complexity, not a replacement for viral load testing.
The Cost and Access Problem
TDM is expensive. In the NHS, a single test costs £250-£350. Insurance in the U.S. often won’t cover it unless it’s tied to a documented interaction or toxicity. Many clinics don’t even offer it because the infrastructure is too heavy. You need trained pharmacists, certified labs, and clinicians who understand pharmacokinetics.
One NHS clinician shared a case: a patient on generic lopinavir/ritonavir kept developing severe nausea and liver enzyme spikes. Viral load was fine. Adherence was good. TDM revealed drug levels were 300% higher than normal. The dose was cut. Symptoms vanished. Without TDM, the patient might have been misdiagnosed with drug resistance and switched to a more toxic, expensive regimen.
But here’s the catch: in one Reddit thread, a patient described waiting six weeks for TDM results. By then, their viral load had already climbed past 10,000 copies/mL. The test came back showing low drug levels-but it was too late. The delay isn’t just inconvenient. It’s dangerous.
Real-World Outcomes: Evidence from the Field
Studies show TDM reduces treatment failure by 15-20% in complex cases. The NHS’s 2022 internal audit of 147 patients found an 18% drop in virologic failure when TDM was used for high-risk scenarios. In Canada, the McGill University Health Centre reports that TDM has helped prevent toxicity in 12% of patients on boosted protease inhibitors.
But the strongest evidence comes from low-resource settings. In South Africa, where generic antiretrovirals make up over 90% of treatment, a pilot program using TDM to monitor patients on generic darunavir cut treatment failure by 22%. That’s not just statistically significant-it’s a public health win.
Still, experts remain divided. The International Antiviral Society-USA says TDM is useful but lacks standardized target ranges for many drugs. The European AIDS Clinical Society recommends it only for select cases. The bottom line? TDM isn’t ready for prime time-but it’s ready for the right patient at the right time.
What Clinicians Need to Know Before Ordering TDM
If you’re considering TDM, here’s what you need to do first:
- Confirm the patient is on an NTI drug (PI or NNRTI)-not an NRTI.
- Rule out adherence issues. TDM won’t fix a patient who skips doses.
- Check for drug interactions. Use resources like the Liverpool HIV Drug Interactions Checker.
- Order the test at the right time: 2-4 hours before the next dose, after at least 3-5 days of stable dosing.
- Partner with a lab that offers rapid turnaround. If results take more than 7 days, reconsider.
- Interpret results with a specialist. A low level doesn’t always mean underdosing-it could mean rapid metabolism.
Training matters. Clinicians need 6-12 months of mentorship to interpret TDM results correctly. That’s why most successful programs tie TDM to infectious disease fellowships or pharmacist-led clinics.
The Future: Targeted Use, Not Routine Screening
TDM won’t become a standard part of every HIV checkup. But its role is growing-especially as more patients take multiple drugs for HIV, hepatitis, TB, and mental health conditions. Newer antiretrovirals like doravirine and cabotegravir are being studied for TDM applicability, especially when combined with strong CYP3A4 inducers like rifampin.
The real future of TDM lies in precision. Not in measuring every patient. But in protecting the ones who need it most: those on generics, those with complex regimens, those with organ damage, those who’ve failed before. In resource-limited settings, TDM could be the key to ensuring generic drugs don’t become a gamble.
It’s not about replacing viral load tests. It’s about adding another layer of safety. For patients on drugs where a few nanograms per milliliter mean the difference between life and relapse, that layer matters.
Is therapeutic drug monitoring used for all HIV drugs?
No. TDM is only useful for drugs with measurable plasma concentrations that directly correlate with effect-mainly protease inhibitors and non-nucleoside reverse transcriptase inhibitors. It does not work for NRTIs (like tenofovir or emtricitabine) because these are prodrugs that must be activated inside cells. Plasma levels of NRTIs don’t reflect their actual activity.
Can TDM detect if a generic drug is inferior to the brand name?
Yes, indirectly. If two patients take the same dose-one on brand, one on generic-and their blood levels differ significantly despite identical dosing and adherence, it suggests the generic isn’t bioequivalent in practice. TDM can flag this issue before viral load rises or toxicity appears.
How long does it take to get TDM results?
In public health systems, results typically take 10-14 days. Private labs in the U.S. can deliver results in 2-3 days for $450-$650. Delays longer than a week can be dangerous, especially if the patient is at risk of treatment failure. Urgent cases should be flagged for expedited processing.
Is TDM covered by insurance?
Coverage varies. In the UK, NHS funds TDM for approved indications like drug interactions or organ impairment. In the U.S., many insurers require prior authorization and only cover it if there’s documented toxicity, non-adherence ruled out, or a known drug interaction. Always check with your payer before ordering.
Can TDM replace viral load testing?
No. TDM measures drug levels in the blood. Viral load measures whether the virus is being suppressed. Both are needed. A patient can have perfect drug levels but still fail treatment due to resistance. Conversely, low drug levels don’t always mean the virus is replicating-adherence or timing issues might be the cause. They’re complementary tools.
Who should order TDM?
TDM should be ordered by clinicians experienced in HIV management-typically infectious disease specialists, pharmacists with HIV training, or clinics with dedicated TDM protocols. General practitioners should refer complex cases to these specialists. Interpreting results requires understanding pharmacokinetics, drug interactions, and patient-specific factors like weight, liver function, and genetics.
What to Do If Your Clinic Doesn’t Offer TDM
If you’re on a generic NTI drug and worried about its performance, start by asking your provider:
- Have you seen any cases where generic versions caused unexpected side effects or treatment failure?
- Can we check for known drug interactions with my other medications?
- Is there a reference lab we can use for TDM if needed?
If your provider says no, ask for a referral to an HIV specialty clinic. Many academic centers offer TDM even if your local hospital doesn’t. In the U.S., centers like the University of California, San Francisco, and the University of Washington have active TDM programs. In Europe, the McGill University Health Centre and several London hospitals offer the service.
Don’t wait for a crisis. If you’re on a complex regimen, especially with generics, TDM might be the quiet safeguard you didn’t know you needed.




Comments (10)
Peter Axelberg
Man, I’ve seen this play out in my clinic. Patient switches to generic darunavir, feels fine for three months, then starts getting dizzy and nauseous. Viral load’s still undetectable, so we chalk it up to stress. Then one day, TDM comes back showing drug levels at 40% of target. Turns out the generic had a different coating that delayed absorption. We bumped the dose, symptoms vanished. No magic, just math. But why does it take six weeks to get results in some places? That’s not healthcare, that’s Russian roulette.
Jennifer Wang
Therapeutic drug monitoring for narrow therapeutic index antiretrovirals remains a critical, albeit underutilized, clinical tool in the management of complex HIV regimens. The pharmacokinetic variability inherent in generic formulations-particularly with protease inhibitors and non-nucleoside reverse transcriptase inhibitors-can lead to subtherapeutic or toxic plasma concentrations despite apparent bioequivalence in regulatory studies. The evidence base, including the South African study cited, demonstrates a clinically significant reduction in virologic failure when TDM is employed in high-risk populations. Standardization of target trough concentrations and broader access to validated assays are necessary to integrate TDM into routine care pathways.
stephen idiado
TDM is a scam. Big Pharma wants you dependent on expensive labs so they can keep selling brand-name drugs. Generics are fine. If your viral load’s suppressed, stop overcomplicating it. Your doctor’s just trying to bill more codes.
Subhash Singh
Could you please clarify the pharmacokinetic parameters used to define the minimum inhibitory concentration for efavirenz in the context of generic formulations? Additionally, are there any published inter-laboratory validation studies for the LC-MS/MS assays employed in TDM for HIV drugs in low-resource settings?
Geoff Heredia
Did you know the FDA doesn’t require generics to prove bioequivalence across all ethnic populations? That’s why Black and Asian patients on generics keep failing. They test on white men, then sell it to everyone. TDM isn’t just helpful-it’s a civil rights issue. And why do private labs charge $650? Because they’re owned by the same companies that make the brand drugs. This isn’t science. It’s a monopoly.
Andrew Keh
I appreciate the depth of this post. TDM isn’t about distrust in generics-it’s about making sure the medicine works for the person taking it. If someone’s on multiple meds, has liver issues, or just isn’t responding, checking drug levels is like checking your tire pressure before a road trip. Simple, smart, and saves lives. Why isn’t this more common?
Peter Lubem Ause
Let me tell you something real-this isn’t just about drugs. It’s about dignity. I’ve seen patients in Lagos on generics who were told, ‘It’s the same thing,’ while their CD4 counts dropped and they couldn’t sleep from nerve pain. TDM doesn’t just measure concentration-it measures whether the system cares enough to look. That one test in South Africa that cut failure by 22%? That’s not data. That’s justice. And if your clinic won’t do it, don’t just accept it. Push. Advocate. Demand. Your life isn’t a cost-saving experiment.
linda wood
So let me get this straight-we’re spending $650 to check if a $5 pill works… but we won’t pay for faster results? And we’re surprised people drop out of care? 😒
LINDA PUSPITASARI
Just had a patient on generic lopinavir with liver spikes-TDM showed 3x normal levels. Cut the dose. Done. No resistance. No new meds. Just science. 🙌 Also, if your lab takes 10 days? Run. Find one that doesn’t. This isn’t optional. It’s survival. And yes I know NRTIs don’t work with TDM-don’t test them please 😅
gerardo beaudoin
My clinic doesn’t do TDM, but we refer to UCSF. Took 4 days to get results. Saved a guy from switching to tenofovir alafenamide when he didn’t need to. Just ask. Don’t assume. It’s not that hard.