For years, treating type 2 diabetes meant one thing: lower blood sugar. Medications like metformin, sulfonylureas, and DPP-4 inhibitors focused almost entirely on HbA1c numbers. But since 2015, everything changed. A single class of drugs - SGLT2 inhibitors - proved they could do more than just manage glucose. They could save hearts. They could protect kidneys. And for many patients, that’s life-changing.
What Are SGLT2 Inhibitors?
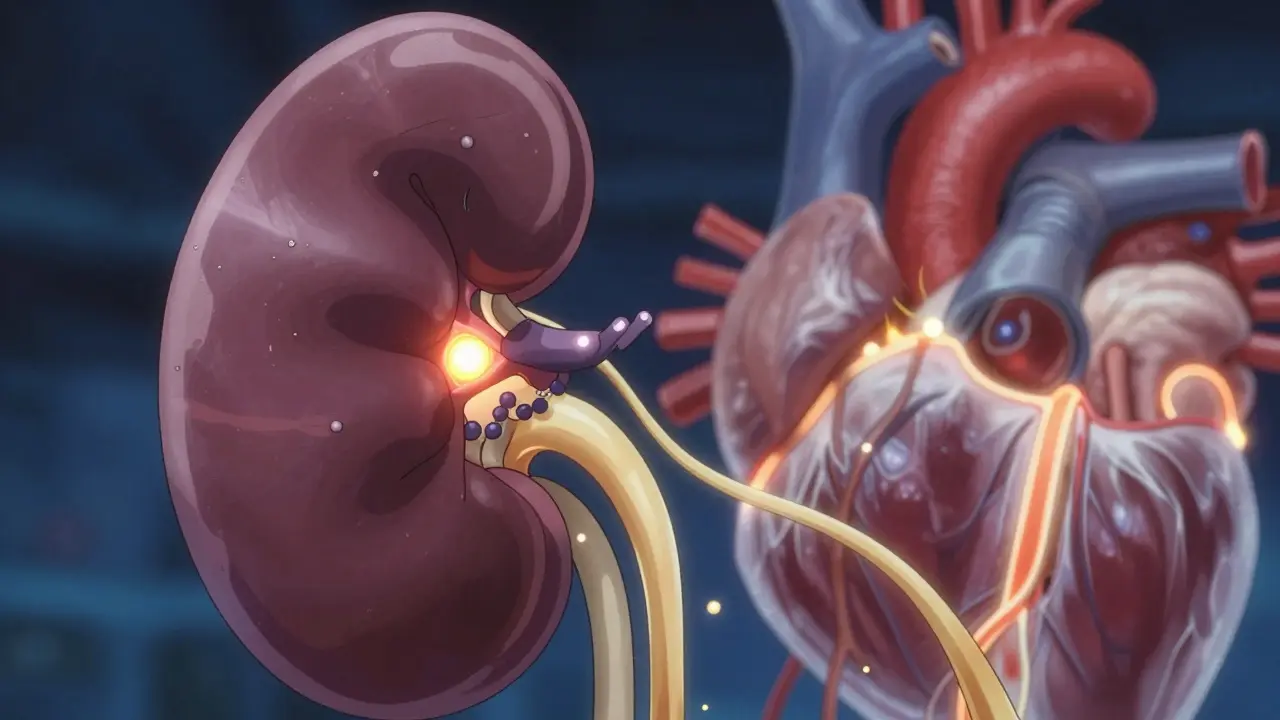
SGLT2 inhibitors - also called gliflozins - are oral diabetes medications that work in a way no other drug class does. Instead of forcing your body to make more insulin or making cells more sensitive to it, they let your kidneys do the work. Specifically, they block a protein called SGLT2 in the proximal tubule of the kidney. This protein normally reabsorbs glucose back into your bloodstream. When it’s blocked, excess sugar flows out in your urine.
That’s why you might notice more frequent urination after starting one of these drugs. It’s not a side effect - it’s the mechanism. Healthy kidneys reabsorb almost all glucose. In type 2 diabetes, that threshold climbs to around 220 mg/dL, meaning your body holds onto sugar even when blood levels are dangerously high. SGLT2 inhibitors reset that threshold to 40-80 mg/dL. Result? You lose 60-90 grams of glucose daily through urine. That’s roughly 240-360 calories. No dieting needed.
The first FDA-approved drug in this class was canagliflozin (Invokana) in March 2013. Since then, four others joined the list: dapagliflozin (Farxiga), empagliflozin (Jardiance), ertugliflozin (Steglatro), and forxiga (the international brand of dapagliflozin). All are taken once daily, with or without food. And while they lower HbA1c by 0.5% to 1.0%, that’s not even the biggest story.
Heart Protection That Changed Medicine
The EMPA-REG OUTCOME trial in 2015 was the turning point. Researchers gave empagliflozin to over 7,000 adults with type 2 diabetes and established heart disease. After three years, those taking the drug had a 38% lower risk of dying from cardiovascular causes. All-cause mortality dropped by 32%. These weren’t small improvements - they were revolutionary.
Before this, no diabetes drug had ever shown such a clear, dramatic reduction in heart-related death. Sulfonylureas? They increase hypoglycemia risk and weight gain. Metformin? Great for glucose and cheap, but no proven mortality benefit. DPP-4 inhibitors? Safe, but neutral on heart outcomes.
Other trials confirmed it. The CANVAS Program showed canagliflozin cut major heart events (heart attack, stroke, death) by 14%. DECLARE-TIMI 58 found dapagliflozin reduced hospitalizations for heart failure by 17%. And the real kicker? These benefits showed up even in patients whose blood sugar didn’t improve much. That meant the heart protection wasn’t just from lower glucose - it was something deeper.
Today, the American Heart Association, the European Society of Cardiology, and the American Diabetes Association all agree: if you have type 2 diabetes and heart failure or heart disease, an SGLT2 inhibitor isn’t optional - it’s standard care. In fact, the 2023 ESC guidelines give them a Class I recommendation - the highest level - for heart failure with reduced ejection fraction, even if you don’t have diabetes.
Kidney Protection: Slowing Down the Silent Killer
Diabetic kidney disease is the leading cause of kidney failure worldwide. It often creeps up without symptoms until it’s too late. That’s where SGLT2 inhibitors made another breakthrough.
The CREDENCE trial in 2019 enrolled 4,400 patients with type 2 diabetes and kidney disease. Those on canagliflozin had a 30% lower risk of reaching end-stage kidney disease, doubling their serum creatinine, or dying from kidney causes. The EMPA-KIDNEY trial in 2023 expanded this even further: empagliflozin reduced major kidney events by 28% in patients with and without diabetes. This was the first time a drug showed kidney protection beyond just controlling blood sugar.
How does this work? It’s not just about less glucose in the blood. SGLT2 inhibitors reduce pressure inside the kidney’s filtering units (glomeruli). This lowers the strain on damaged kidney tissue. Think of it like turning down the water pressure in a leaking pipe - it doesn’t fix the crack, but it slows the leak. That’s why many patients see a small dip in eGFR (a measure of kidney function) early on. It’s not damage - it’s the drug doing its job. The decline usually stabilizes after 2-3 months, and long-term kidney function improves.
Now, the American Society of Nephrology recommends starting SGLT2 inhibitors in patients with chronic kidney disease and albuminuria (protein in urine), even if they don’t have diabetes. That’s how powerful this class has become.

Real Patient Experiences
People aren’t just seeing numbers change - they’re feeling better.
On patient forums, common reports include:
- Weight loss of 5-12 pounds in the first few months
- Lower blood pressure (3-5 mmHg systolic drop)
- More energy and less fatigue
- Improved heart failure symptoms - like less shortness of breath
But there are trade-offs. Genital yeast infections are the most common side effect, affecting 4-5% of users. That’s because sugar in urine creates a breeding ground for fungi. It’s treatable, but frustrating. Some report increased urination at night, which can disrupt sleep. And while rare, diabetic ketoacidosis (DKA) can happen - even when blood sugar isn’t high. This is called euglycemic DKA, and it’s dangerous because it’s easy to miss. Symptoms: nausea, vomiting, abdominal pain, confusion. If you’re sick, fasting, or having surgery, talk to your doctor about temporarily stopping your SGLT2 inhibitor.
Canagliflozin carries a boxed warning for increased risk of lower-limb amputations, especially in people with prior foot ulcers or peripheral artery disease. That’s why doctors screen carefully before prescribing.
Who Should Take Them? Who Should Avoid?
These drugs are now recommended for:
- Type 2 diabetes patients with heart failure (any ejection fraction)
- Patients with established cardiovascular disease
- Those with chronic kidney disease (eGFR ≥25, albuminuria >30 mg/g)
- People with obesity or hypertension who need additional glucose control
They’re not for everyone:
- Not approved for type 1 diabetes
- Avoid if eGFR is below 25-30 mL/min (varies by drug)
- Use caution in elderly patients or those on diuretics - risk of low blood pressure or dehydration
- Not recommended if you have a history of recurrent genital infections
Doctors now start these drugs earlier - often alongside metformin, not after. The American Diabetes Association’s 2023 guidelines say: if you have heart or kidney disease, start with an SGLT2 inhibitor or GLP-1 RA. Metformin is still first-line for people without those conditions, but the tide has turned.
Cost and Accessibility
These drugs aren’t cheap. Brand-name versions cost $520-$600 per month before insurance. Generic versions aren’t available in the U.S. yet - patents expire between 2025 and 2028. Many patients struggle with coverage. Some insurers require step therapy - trying metformin first - even when guidelines say otherwise.
But the long-term savings are real. A 2022 analysis showed SGLT2 inhibitors cost about $38,400 per quality-adjusted life year gained - well under the $50,000 threshold used in U.S. healthcare. Fewer hospital stays for heart failure and kidney dialysis mean lower overall costs for the system. As generics arrive, access will improve dramatically.
What’s Next?
Research is expanding fast. The DELIVER trial showed dapagliflozin helps people with heart failure and preserved ejection fraction (HFpEF) - a condition with almost no effective treatments. The SUGAR-DM trial is exploring whether ketone bodies - produced when the body burns fat - are the real reason these drugs protect the heart and kidneys.
By 2027, the global market for SGLT2 inhibitors is projected to hit $18.5 billion. That’s because they’re no longer just diabetes drugs. They’re cardiorenal metabolic drugs. They’re changing how we treat not just diabetes, but the whole cluster of conditions that come with it: obesity, high blood pressure, heart failure, and kidney disease.
The message is clear: if you have type 2 diabetes and heart or kidney issues, ask your doctor about SGLT2 inhibitors. They’re not just another pill. They’re one of the most important advances in diabetes care in decades.
Do SGLT2 inhibitors cause weight loss?
Yes. On average, patients lose 2-3 kg (4-7 lbs) in the first few months, mostly from water and fat. The mechanism is simple: you’re losing glucose (and calories) through urine. This weight loss is sustained over time and is one reason these drugs are now preferred for people with obesity and type 2 diabetes.
Can I take SGLT2 inhibitors if I have kidney disease?
Yes - and you should. Unlike many diabetes drugs, SGLT2 inhibitors are safe and effective even in early to moderate kidney disease (eGFR ≥25 mL/min). They actually slow progression of kidney damage. The FDA has approved dapagliflozin and empagliflozin for chronic kidney disease, even without diabetes. Your doctor will monitor your eGFR and adjust the dose if needed.
Why do I keep getting yeast infections?
Sugar in your urine creates a warm, moist environment where yeast thrives. This is the most common side effect, affecting 4-5% of users. It’s more common in women but can happen in men too. Treatments like antifungal creams or oral fluconazole work well. Drinking more water and keeping the area clean helps prevent recurrence. If infections keep coming back, talk to your doctor about switching drugs.
Can SGLT2 inhibitors cause diabetic ketoacidosis?
Yes, but it’s rare - about 0.1-0.3% of users. This is called euglycemic DKA, meaning your blood sugar may only be mildly high (100-250 mg/dL), making it harder to spot. Symptoms include nausea, vomiting, abdominal pain, fatigue, and confusion. If you’re sick, having surgery, or not eating, stop your SGLT2 inhibitor and contact your doctor immediately. Always check ketones if you feel unwell while on these drugs.
Are SGLT2 inhibitors better than GLP-1 agonists?
They’re different, not better. Both protect the heart and kidneys. GLP-1 agonists (like semaglutide) are stronger for weight loss and HbA1c reduction. SGLT2 inhibitors are better for reducing heart failure hospitalizations and are easier to take (pill vs. injection). Many doctors now use them together for maximum benefit - especially in high-risk patients. Your choice depends on your health goals, preferences, and insurance coverage.




Comments (8)
Siobhan K.
Let’s be real - this class of drugs didn’t revolutionize diabetes care because of some magic bullet. It was because the pharmaceutical industry finally stopped pretending that lowering HbA1c was the only metric that mattered. The fact that it took 30 years and thousands of deaths for guidelines to catch up says more about medical inertia than it does about science.
I’ve seen patients on metformin for a decade with perfect A1cs but still ending up in heart failure. Meanwhile, the same patients on dapagliflozin? Less hospital visits, better sleep, no more swollen ankles. The numbers don’t lie - it’s just that most doctors still treat diabetes like it’s a sugar problem, not a systemic metabolic disaster.
And don’t get me started on the insurance hurdles. I had to fight for six months to get my patient approved for Jardiance. They wanted her to try three other drugs first. Three. While her kidneys slowly died.
Yes, yeast infections suck. Yes, DKA is scary. But the risk-benefit ratio is so skewed toward benefit that it’s almost unethical not to prescribe these to high-risk patients. The real tragedy isn’t the side effects - it’s the patients who never even hear about this option until it’s too late.
Brian Furnell
As a nephrologist, I can confirm: the renal protective effects of SGLT2 inhibitors are among the most robust findings in modern clinical medicine. The hemodynamic shift - reduced intraglomerular pressure via tubuloglomerular feedback modulation - is not just theoretical; it’s measurable via renal biopsy and longitudinal eGFR trajectories.
What’s remarkable is that this effect persists even in non-diabetic CKD, which fundamentally redefines the therapeutic target: it’s not hyperglycemia per se, but rather the maladaptive renal response to metabolic stress. The EMPA-KIDNEY trial was the first to demonstrate this in a truly generalizable population.
And yes, the initial dip in eGFR? That’s not toxicity - it’s autoregulation. I’ve had patients panic when their creatinine rose 0.3 mg/dL on day 30 - only to stabilize at a 15% higher eGFR than baseline after 12 months. This isn’t a side effect - it’s a biomarker of therapeutic efficacy.
Still, we’re not out of the woods. Long-term data beyond five years is sparse, and we still don’t fully understand the role of ketone body utilization in cardioprotection. But for now? This is the closest thing we have to a disease-modifying therapy in nephrology.
Jason Silva
EVERYTHING ABOUT THIS IS A PHARMA LIE 😤
They’re not saving hearts - they’re making you pee out sugar so your body burns fat and you lose weight. That’s it. No magic. Just a fancy diuretic with a side of ketoacidosis risk. The ‘heart protection’? It’s just from weight loss and lower BP. They didn’t test these drugs against a low-carb diet. Why? Because Big Pharma doesn’t want you eating avocado and eggs.
And don’t even get me started on the amputation warning. That’s not a side effect - it’s a cover-up. These drugs cause poor circulation in the feet because they dehydrate you. The FDA knows. They just don’t want to scare people away from their $600/month pills.
Also - why do you think the trials only include people with existing heart disease? Because they needed a population that would die fast enough to show a difference. They didn’t test it on healthy people. Why? Because if it worked for prevention, they’d have to give it to everyone. And then… no more profits.
💀🩸💊
mukesh matav
I’m from India, and we don’t have access to these drugs easily. Even if we do, they’re unaffordable. My uncle has diabetes and kidney issues - he’s on metformin and insulin. He can’t even afford the monthly checkups, let alone a $600 pill.
I’m glad these drugs work, but it feels wrong to celebrate them when most people in the world will never see them. Maybe the real breakthrough should be making them cheap and accessible - not just scientifically impressive.
Also, the yeast infections… yeah, that’s real. My cousin had to stop it after three months. She said it was worse than the diabetes.
Peggy Adams
Okay but why do I have to take yet another pill? I’m already on metformin, a statin, and a blood pressure med. Do I really need a fourth thing that makes me pee like a racehorse and gives me yeast infections? 🤮
I’d rather just cut out the bread and sugar and move more. But nooo, the system wants me on five drugs and a wearable. I’m tired.
Sarah Williams
This is the most hopeful thing I’ve read about diabetes in years. Seriously. If you have heart or kidney issues and diabetes - ask your doctor. Don’t wait. Don’t assume metformin is enough. These drugs are life-changing. I’ve seen it firsthand. One patient went from needing oxygen to walking her dog daily in 3 months. That’s not a miracle - it’s science. 💪❤️
Theo Newbold
Let’s dissect the data. The EMPA-REG trial showed a 38% reduction in CV death - but the absolute risk reduction was 2.5%. That’s 40 patients needed to treat for three years to save one life. Meanwhile, the cost per QALY is $38k - above the $25k threshold used in many European health systems.
The yeast infection rate is 5%. The DKA risk is low but fatal. The amputation risk with canagliflozin is real - and it’s not just correlation. The mechanism? Volume depletion → reduced perfusion → tissue necrosis.
These are not ‘miracle drugs.’ They’re high-risk, high-cost interventions with marginal population-level benefit. They’re appropriate for select patients - not a universal recommendation. The guidelines have become marketing documents.
Jackie Be
I started Jardiance last year and my energy is UNREAL like I’m not dragging myself through the day anymore and I lost 8 lbs without trying and my A1c dropped from 8.2 to 6.9 and my doc said my kidneys are doing better but then I got a yeast infection so bad I cried and now I’m scared to take it but I don’t wanna go back to feeling like a zombie so I’m just gonna keep taking it and pray 🙏