When you pick up a prescription at the pharmacy and see a cheaper version of your brand-name drug, you’re holding a product cleared by the FDA through one of the most precise and science-driven approval systems in the world. This isn’t just a label swap-it’s a rigorously controlled process designed to ensure safety, effectiveness, and affordability. The FDA doesn’t just rubber-stamp generics. It demands proof-down to the last milligram-that the generic works exactly like the brand-name drug. And it’s this system that keeps 9 out of 10 prescriptions in the U.S. affordable.
How the FDA Approves Generic Drugs
The FDA doesn’t require generic manufacturers to repeat the expensive clinical trials that proved the original drug was safe and effective. Instead, they use a streamlined pathway called the Abbreviated New Drug Application, or ANDA. This process was created by the Hatch-Waxman Act of 1984 to balance innovation with access. The goal? Let generics enter the market faster and cheaper-without cutting corners on quality.
To get approval, a generic drug must match the brand-name drug in four key ways: the same active ingredient, same strength, same dosage form (like tablet or injection), and same route of administration (oral, topical, etc.). It must also have the same intended use. Inactive ingredients-like fillers or dyes-can differ, but they can’t affect how the drug works in the body.
Bioequivalence: The Science Behind the Swap
The core of the ANDA process is bioequivalence. This means the generic drug must be absorbed into the bloodstream at the same rate and to the same extent as the brand-name version. The FDA doesn’t guess-it measures.
Manufacturers run studies with 24 to 36 healthy volunteers. Blood samples are taken over time to track how much of the drug enters the bloodstream and how quickly. The data is analyzed to calculate two key values: AUC (total exposure) and Cmax (peak concentration). For approval, the 90% confidence interval for both must fall between 80% and 125% of the brand-name drug’s values. That’s not a wide margin-it’s a tight scientific standard.
For complex drugs like inhalers, patches, or extended-release tablets, the rules get even stricter. The FDA requires specialized testing methods, sometimes including clinical endpoint studies, because absorption can’t be measured just by blood levels. In 2023, nearly 38% of approved generics fell into this complex category-a big jump from 22% in 2018.
The Review Process: From Submission to Approval
Submitting an ANDA isn’t just filling out forms. It’s compiling 15,000 to 20,000 pages of data on chemistry, manufacturing, controls, and bioequivalence studies. The FDA’s Office of Generic Drugs (OGD) reviews every submission.
First, the application goes through a Filing Review. If it’s missing critical information-like incomplete manufacturing details-it gets a Refuse-to-Receive (RTR) letter. In 2022, 15.3% of applications were rejected at this stage. Many of these failures came from poor documentation of how the drug was made, not from safety issues.
Once filed, the review clock starts. Standard applications have a 10-month target. Priority applications-like first generics or drugs in short supply-get an 8-month deadline. In 2023, the FDA approved 1,256 ANDAs, up 12.7% from the year before. But they also issued 317 RTR letters, showing how easy it is to get tripped up by paperwork.
Manufacturing: Where Quality Is Built
A generic drug can be perfectly bioequivalent, but if it’s made in a dirty or poorly controlled facility, it’s dangerous. That’s why the FDA inspects every manufacturing site-whether it’s in the U.S., India, or China.
Every facility must follow Current Good Manufacturing Practices (CGMP), which cover everything from equipment cleaning to employee training. In 2023, the FDA inspected 82.7% of generic drug plants annually. If a facility fails inspection, the application is put on hold. No exceptions.
And it’s not just about inspections. The FDA also requires manufacturers to submit detailed plans for how they’ll control quality over time. This includes stability testing to prove the drug won’t break down in heat or humidity before its expiration date.
Costs and Barriers for Manufacturers
Developing a generic drug costs between $2.4 million and $6.3 million-far less than the $2.6 billion it takes to bring a new drug to market. But the financial and technical hurdles are still high.
Each ANDA application costs $389,490 in user fees under GDUFA III, plus annual facility fees ranging from $207,700 to $415,400. Smaller companies often struggle with these costs. Many hire teams of 8 to 12 specialists-regulatory experts, pharmacologists, chemists-to navigate the process.
First-time applicants typically need 18 to 24 months to get it right. Common mistakes? Poorly designed bioequivalence studies and CGMP violations. In fact, 41.7% of RTR letters cited manufacturing documentation issues. The FDA offers pre-ANDA meetings to help companies avoid these pitfalls. In 2022, 78.4% of approved applications had used this service.
Why This System Works
The result? Generics make up 90% of U.S. prescriptions but only 23% of total drug spending. In 2023 alone, they saved the healthcare system $132.6 billion. For patients, that means insulin dropping from $390 a month to $98. Blood pressure pills, antibiotics, antidepressants-all cheaper because the FDA made sure they’re just as good.
Patients trust the system. A 2023 CVS survey found 78.4% of users felt confident in generic drugs. Sixty-three percent reported no difference in how they felt compared to the brand-name version.
Even when patients report issues-like a generic not working as well-the FDA investigates. Between 2020 and 2023, there were 1,485 adverse event reports involving generics. But 92.3% of those cases were traced to the patient’s condition worsening, not the drug itself.
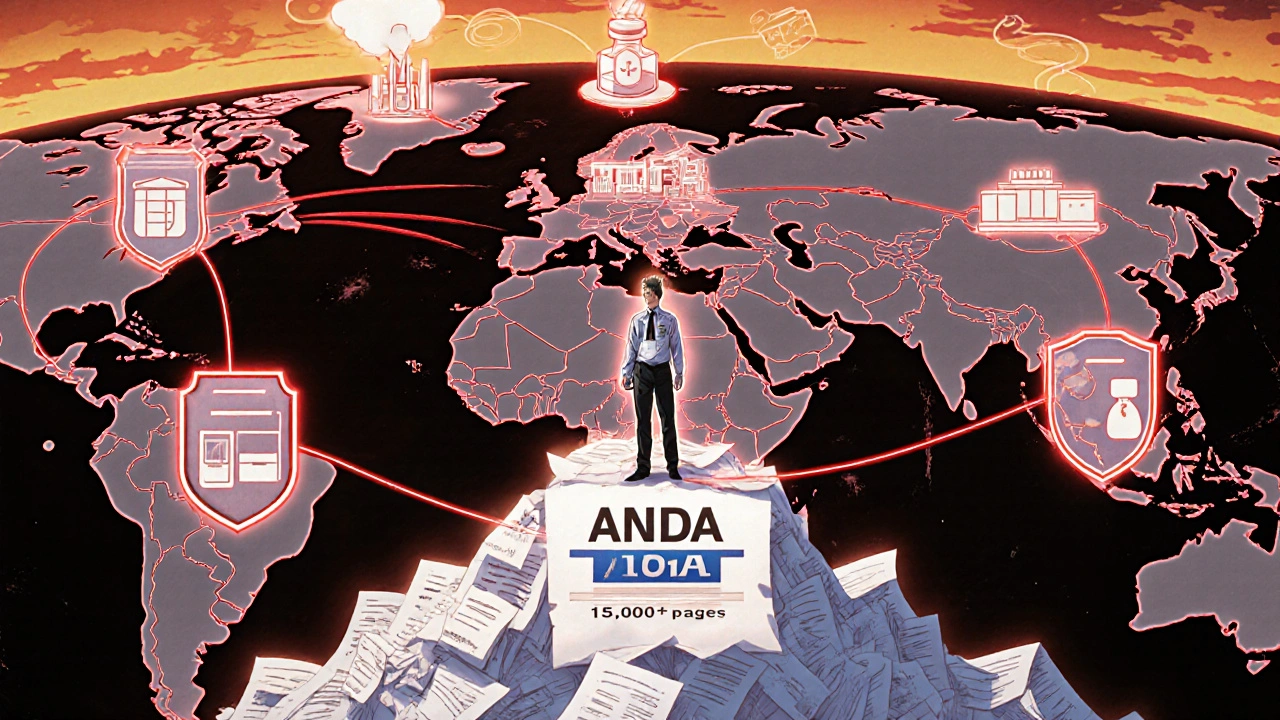
Challenges and Criticisms
It’s not perfect. In 2022, Senator Bernie Sanders’ committee found 1,842 ANDAs were still pending, with 317 stuck for over three years. The FDA admitted that 14.8% of applications received complete response letters due to bioequivalence study flaws.
Supply chain risks are another concern. Nearly 78% of active ingredients for generics come from outside the U.S.-mostly India and China. The FDA is working to strengthen oversight, but global dependency remains a vulnerability.
Patent litigation also slows things down. On average, each branded drug facing generic competition faces 34.7 patent challenges. The FDA’s Drug Competition Action Plan has helped cut approval times for first generics from 42.1 months to 26.4 months since 2017.
What’s Next for Generic Approval
The FDA is pushing forward. In October 2025, they launched a pilot program to speed up reviews for generics made in the U.S. Applications from domestic manufacturers now get a 30% faster review clock.
Under GDUFA IV, which runs through 2027, $412 million is being invested specifically for complex generics. The agency is also testing AI tools to help review applications faster and exploring how real-world data-like electronic health records-can support approval decisions.
By 2027, analysts predict the FDA will approve 1,500 to 1,700 generic applications annually. With over 2,100 first generics already in the queue, the pressure to keep up is real. But the system is adapting.
What You Should Know as a Patient
If your doctor prescribes a generic, it’s not a compromise-it’s science-backed savings. The FDA ensures it’s the same drug, just cheaper. You don’t need to worry about effectiveness. If you notice a change in how you feel after switching, talk to your pharmacist or doctor. But don’t assume the generic is the problem. In most cases, it’s not.
Check the FDA’s Orange Book to see if your drug has been approved as therapeutically equivalent. And remember: every time you choose a generic, you’re helping lower costs for everyone.
Are generic drugs as effective as brand-name drugs?
Yes. The FDA requires generics to be bioequivalent to their brand-name counterparts, meaning they deliver the same amount of active ingredient into the bloodstream at the same rate. Studies show 92% of patients report no difference in effectiveness after switching. The FDA reviews thousands of bioequivalence studies each year to confirm this.
Why do some generics look different from the brand-name drug?
The active ingredient must be identical, but inactive ingredients-like color, shape, flavor, or fillers-can differ. These changes don’t affect how the drug works. Differences in appearance are often due to trademark laws that prevent generics from looking exactly like the brand-name version.
How long does it take for the FDA to approve a generic drug?
Standard ANDA applications have a 10-month review target. Priority applications-such as first generics or drugs in shortage-are reviewed in 8 months. But the timeline can extend if the application is incomplete or requires additional data. In 2023, 1,256 generics were approved, but 317 submissions were refused at filing due to missing information.
Are all generic drugs made in the U.S.?
No. About 78% of active pharmaceutical ingredients for generics come from facilities outside the U.S., mostly in India and China. The FDA inspects all facilities-domestic or foreign-that supply drugs to the U.S. market. Every plant must meet the same quality standards, regardless of location.
Can a generic drug be pulled from the market after approval?
Yes. If post-market surveillance finds safety issues, manufacturing violations, or evidence the drug isn’t bioequivalent, the FDA can withdraw approval. This has happened in rare cases, often due to contamination or falsified data. The FDA monitors adverse events through its FAERS database and conducts follow-up inspections when needed.





Comments (14)
Michael Fessler
Man, the bioequivalence standards are insane. 80-125% confidence interval on AUC and Cmax? That’s tighter than a drum. I’ve seen people freak out because their generic pill is a different color, but the science? Rock solid. The FDA doesn’t wing it-they measure blood levels like it’s a damn physics lab. And yeah, the manufacturing inspections? 82.7% annual checks? That’s not oversight, that’s a full-time surveillance operation. No wonder so many apps get RTR’d-half the time it’s just sloppy documentation, not bad drugs.
daniel lopez
Let me guess-this is all just a corporate shill piece. FDA? More like FDA-Pharma-Industrial Complex. You think they really inspect every plant in India and China? LOL. They get paid by the drug companies. You think they’d shut down a factory that makes 78% of the world’s active ingredients? Nah. They just slap a warning letter on it and call it a day. And don’t even get me started on ‘first generics’-those are always delayed until the brand-name patent expires by 3 weeks. Coincidence? I think not.
Nosipho Mbambo
Okay, so… generics are cheap, right? And the FDA says they’re the same? But… what if they’re not? I mean, I switched to a generic for my blood pressure med and I felt… weird. Like, dizzy. And then I switched back and boom-fine. So… what’s the deal? Are we just supposed to trust this? It’s like… a whole system built on faith. And I’m not feeling it. 😐
Alyssa Torres
Y’ALL. I just want to say-this is one of the most important things you’ll ever read about healthcare. 💗 The fact that someone in rural Mississippi can get insulin for $98 instead of $390? That’s not magic. That’s the FDA doing its damn job. And yes, I know people say ‘but my pill looks different!’-but guess what? So does my coffee mug when I buy a new brand. Doesn’t mean it’s not coffee. This system saves lives. Every. Single. Day. Stop being scared of a different color pill and start being grateful for the science that makes it possible.
Summer Joy
OMG I CAN’T BELIEVE YOU’RE STILL BUYING THIS!!! 😭 The FDA is a puppet! I saw a TikTok that said 40% of generics are just sugar pills with a fancy label!! And they’re made in basements in China!! I mean… LOOK AT THE NUMBERS!! 1,256 approved in 2023? That’s a MASSIVE increase!! That’s not progress-that’s a collapse!! 💀
Aruna Urban Planner
The beauty of the ANDA system lies in its balance-not between profit and public health, but between rigor and pragmatism. The science of bioequivalence is not merely statistical; it’s physiological. The 80-125% range isn’t arbitrary-it’s derived from pharmacokinetic variability in human populations. What’s often missed is that this framework allows innovation in delivery systems-like extended-release patches-without requiring full clinical trials. That’s not laziness. That’s intelligent regulation.
Nicole Ziegler
generic = same drug, cheaper price 😎 I’ve switched 5 times. Never noticed a difference. Also, my dog takes generic heartworm med and he’s still alive. So… yeah. 🐶💊
Bharat Alasandi
bro the real win here is that a guy in Mumbai can make a pill that’s just as good as the one in NYC and the FDA still checks it. That’s global science working. And yeah, the fees are high-but imagine trying to get this right without specialists. It’s like trying to build a rocket with a manual from 1998. The pre-ANDA meetings? Lifesavers. The system’s flawed? Sure. But broken? Nah. It’s working.
Kristi Bennardo
This entire post is a dangerous illusion. The FDA is not a guardian of public health-it is a regulatory arm of Big Pharma. The 15,000-page applications? Designed to exclude small manufacturers. The 317 RTR letters? A deliberate bottleneck. And let’s not forget: the agency’s funding is tied to industry user fees. This is not oversight-it is capture. If you believe generics are safe, you believe in fairy tales written by lawyers.
Shiv Karan Singh
HAHAHAHA. 90% of prescriptions are generics? So what? That means 10% are brand-name-and those are the ones the rich people take. The rest of us are just guinea pigs for the FDA’s ‘close enough’ policy. And you think 80-125% is tight? That’s a 45% range! I could take a pill that’s half as strong and it’d still be ‘approved.’ You’re all sheep. 🐑
Ravi boy
u forgot to mention that indian factories are inspected more than some us schools. and most generics are made in india. and yes the fda checks em. and yes they shut em down. i saw a news clip last year where a plant got banned for falsifying data. its not perfect but its better than you think. also-generic metformin saved my dad’s life. so yeah.
Matthew Karrs
Let’s be real-how many of these ‘approved’ generics have been recalled? How many patients have had seizures or strokes because of a bad batch? The FDA doesn’t track outcomes, only paperwork. And the ‘adverse events’ reports? They’re underreported by 90%. This whole thing is a house of cards built on trust and ignorance.
Matthew Peters
So… the FDA approves 1,256 generics a year, but 317 get refused at filing? That’s like a 20% failure rate before they even start reviewing. That’s wild. It means most companies just throw stuff at the wall and hope it sticks. And the ones that do make it? They’re probably cutting corners on stability testing. I mean… who’s checking if that pill still works after 2 years in a hot garage? 🤔
Liam Strachan
Really appreciate this breakdown. I’m from the UK and we have a similar system here (MHRA), but it’s fascinating to see how detailed the FDA process is. The bioequivalence thresholds are impressively strict-I’d love to see more countries adopt this level of rigor. And props to the inspectors flying to India and China to check facilities. That’s not easy work. The system isn’t perfect, but it’s one of the best we’ve got.